January 2025

We are very happy to announce that we recruited 2000 out of the intended 3000 inclusions!
May 2024
We are very happy to announce a change in the inclusion criteria for the TUBA-WISP II study. From this point forward, also PALB2 PV carriers can be included. The other included pathogenic variants remain the same: BRCA1, BRCA2, RAD51C, RAD51D and BRIP1.
The lifetime risk for ovarian cancer with a PALB2 PV ranges from 3-5% and a risk-reducing surgery can be considered when the lifetime risk is >5%. Individual factors influence the personal risk assessment, and therefore we decided to also include the PALB2 PV carriers that are eligible for risk-reducing surgery. We recommend to consider for every individual patient whether or not the benefits of risk-reducing surgery outweigh the risks. An individual risk assessment tool could be used to quantify the ovarian cancer risk (like CanRisk). This does not apply to all participating centers.

March 2024
At the European Society for Gynaecological Oncology conference (ESGO) we held the TUBA-WISP II invesitgators meeting! We were very happy to welcome a lot of the participating centres and to discuss the study with everyone.

March 2024
We are very happy to announce that we are halfway with our inclusions! We've currently reached 1500 out of the intended 3000 inclusions!

Newsletter March 2024


Newsletter December 2023


Newsletter September 2023

Newsletter November 2022

Newsletter June 2022

Newsletter March 2022

Newsletter December 2021
Newsletter September 2021

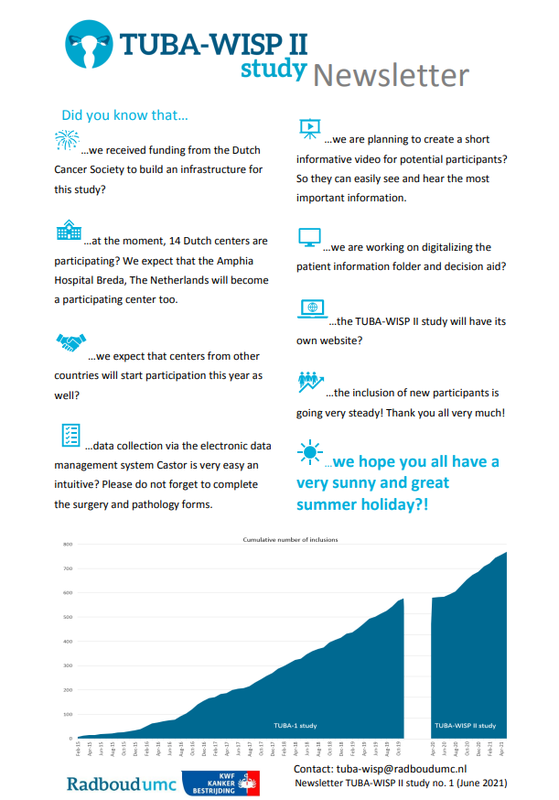
Newsletter June 2021